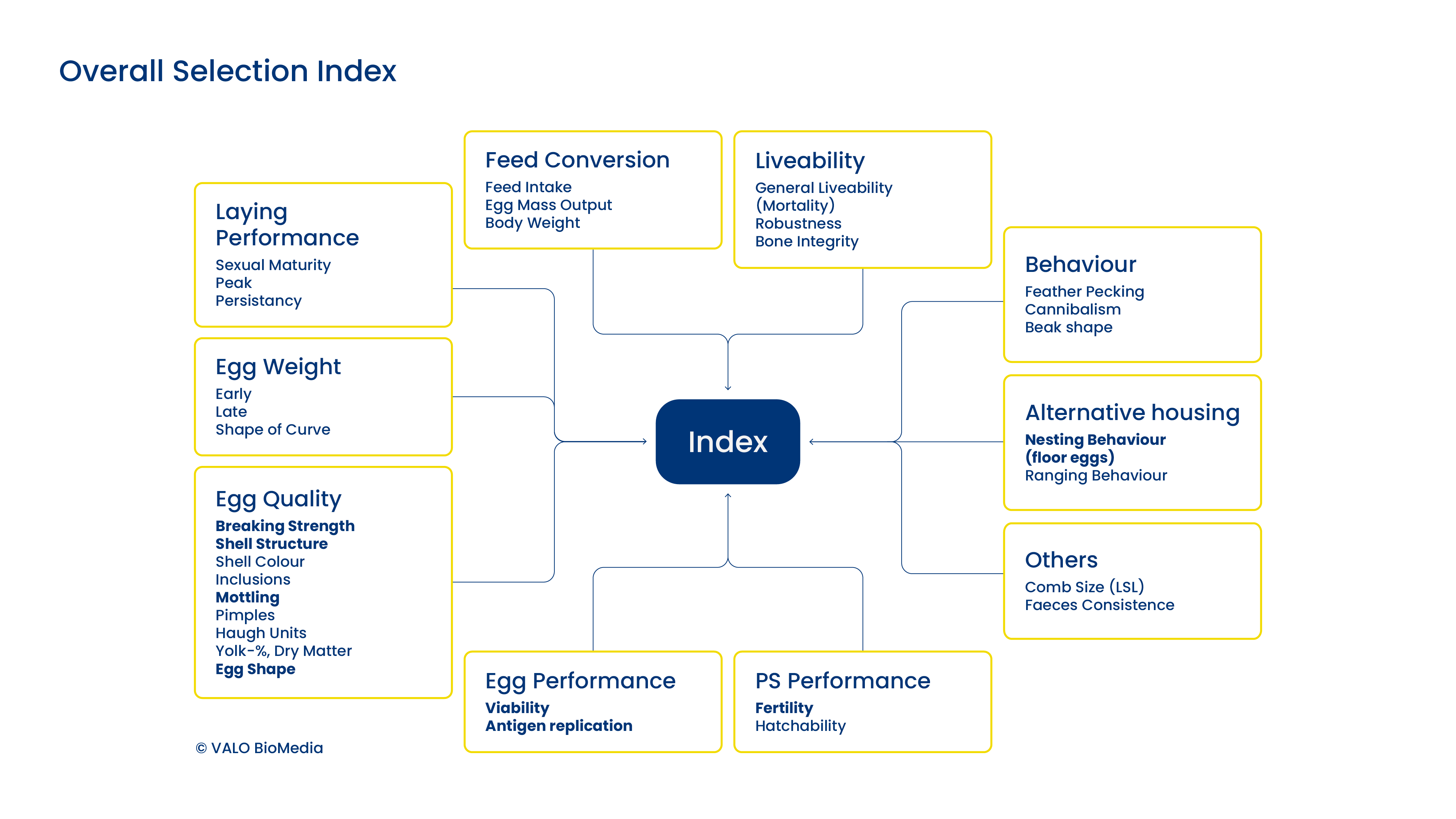
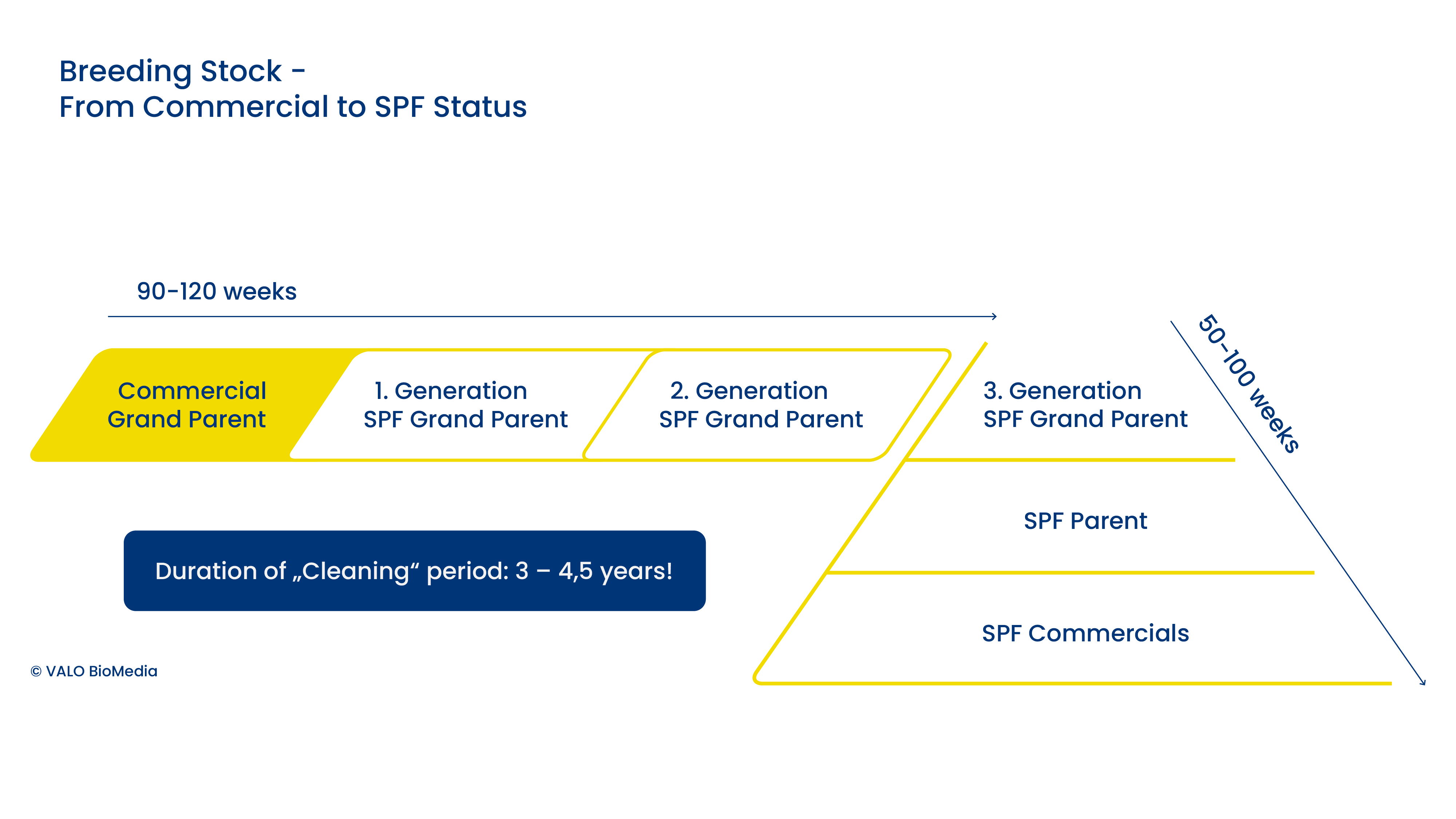
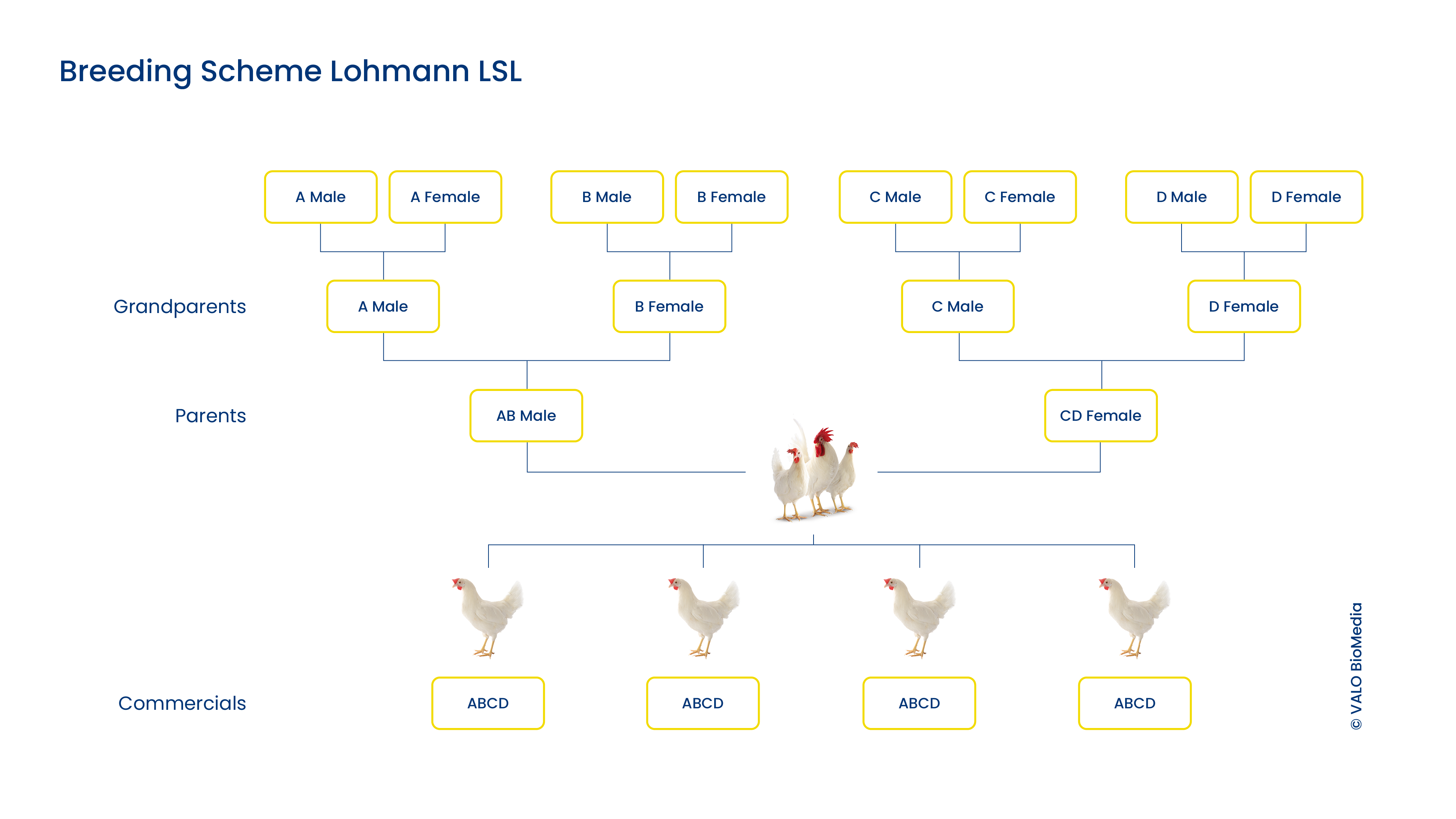
Genetics

Better vaccine yield
Success monitoring
We record all our production data digitally and in detail worldwide. This gives us a detailed view of the performance of the animals in production as well as selected quality parameters of the SPF eggs (such as fertilization rates, shell stability).

In addition, we receive structured feedback from our customers on the results the eggs have yielded in vaccine production or research trials.
Based on the data collected internally and externally, we discuss with the geneticists at Lohmann Breeders what the breeding index for the next generation of SPF genetics should look like.
We repeat this cycle every 2 years so that refreshed genetics are available for production every 2 years as well.
Feed
High-quality feed is the basic prerequisite for the health of the animals and for them to be able to reach their genetic performance potential. The composition of the feed according to the age of the animals is also a decisive factor in ensuring that the eggs meet the set requirements in terms of the following factors: size, weight, shell stability, and shell structure.
Furthermore, in the SPF area, feed represents a significant risk for the introduction of undesirable pathogens. To minimize this risk, we rely on our own feed mill to supply our SPF stocks in Europe. It is located in the north of Germany and enables us to achieve the best possible feed safety and quality through the selection of raw material quality, thermal treatment, and all associated delivery and distribution procedures. This is also documented by the certifications according to GMP+ B1, HACCP, ISO 9001:2015.
We ensure equally high feed quality and safety for our SPF animals in the U.S. and Brazil through feed mills operated by our sister company at the highest level of hygiene.
In the area of clean egg production, we work with external, local feed mills that are first approved by us through audits and then regularly audited.

Infrastructure and processes

We continuously invest in both the physical and IT infrastructure of our production. This applies to all locations and products and is carried out with the aim of high-quality and efficient production with the best biosecurity and the highest animal welfare standards.
Regular investments are also made in the continuous further development of disinfection, sorting, incubation and distribution processes at all locations. In this way, we make each of these processes more sustainable and more efficient in terms of higher vaccine yield.


The starting point for any further development is the live data that we continuously generate and evaluate in our production processes. They give us the opportunity to identify and realize potential for improvement in the production process at an early stage. We incorporate our customers' data to ensure a comprehensive assessment of the process chain. Because we are pursuing the common goal of being able to produce more vaccine doses with fewer eggs in the end.